Developing ozone hole approaches Scandinavia and Eastern Europe right now
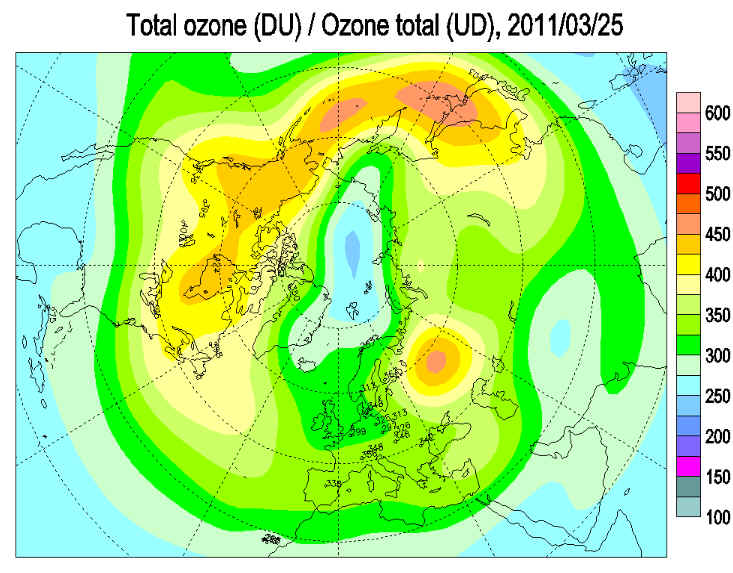
An exceptionally large area of depleted ozone has formed over the North Pole, and scientists warn that it could settle over Scandinavia and Eastern Europe on 30-31 March.
The fast-thinning Arctic ozone layer was Alfred Wegener Institute for Polar and Marine Research. The institute’s latest predictions, based on data collated from the European Centre for Medium Range Weather Forecasts, are that it will affect parts of Scandinavia and Eastern Europe on 30 March and 31 March.

“The ozone loss is still going on at high rates and we don’t see an end to that for at least 10-14 days,” Markus Rex, an atmospheric scientist at the Alfred Wegener Institute. “The degree of ozone loss in the Arctic is clearly larger than in any winter so far.“
Scientists claim that the ozone loss is still going on at high rates and they don’t see an end to that for at least 10-14 days. Polar station measurements showed that around half of the ozone had been destroyed at some latitudes and the Arctic was on track for a record loss of ozone, which protects earth from ultra-violet (UV) radiation.
Ultra-violet radiation (UV) exposure from the depleted ozone layer is less dangerous than that found in the tropics, but scientists still advised caution. Scientists advice that people should be vigilant but they shouldn’t be worried. They suggest that enyone can protect himself by keeping informed, putting on a wide-brimmed hat and sun screen, and not spending too many hours outdoors. A “severe depletion” of ozone was taking place because “this is among the most severe winters we have seen“. But it was still too soon to say if it would be the worst ever.

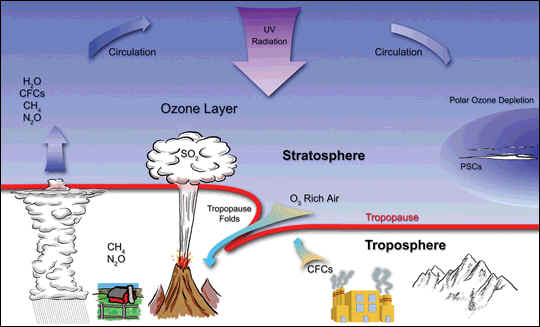
The destruction of the Earth’s ozone is caused by a chemical reaction between atomic chlorine and bromine that takes place in the stratosphere at the sort of very low temperatures most commonly found in the Antarctic. These halogen and halon atoms entered the atmosphere in the form of man-made chlorofluorocarbons (CFC‘s) which were commonly used in appliances such as aerosols, flame retardants and refrigerants from the 1920s onwards.
Chlorofluorocarbons were banned by the Montreal Protocol in 1987. Most man-made ozone depleting substances are also very potent greenhouse cases, notes the European Commission, with some of them 14,000 times stronger than CO2. Therefore, eliminating those substances also contributes significantly to the prevention of climate change, it says.
However, the Commission admits that “much remains to be done to ensure the continuous recovery of the ozone layer and to reduce the impact of ozone depleting substances on climate change“.
Polar station measurements showed that around half of the ozone had been destroyed at some latitudes and the Arctic was on track for a record loss of ozone, which protects earth from ultra-violet (UV) radiation. Data is still being collated and a collection of atmospheric scientists from around the world plan to release a statement on the phenomenon at a conference in Vienna on 4 April.
Developing ozone hole approaches Europe (EurActiv)
What is OZONE?
Ozone is a colorless gas. Chemically, ozone is very active; it reacts readily with a great many other substances. Near the Earth’s surface, those reactions cause rubber to crack, hurt plant life, and damage people’s lung tissues. But ozone also absorbs harmful components of sunlight, known as “ultraviolet B”, or “UV-B”. High above the surface, above even the weather systems, a tenuous layer of ozone gas absorbs UV-B, protecting living things below.
What is OZONE HOLE?
Each year for the past few decades during the Southern Hemisphere spring, chemical reactions involving chlorine and bromine cause ozone in the southern polar region to be destroyed rapidly and severely. This depleted region is known as the “ozone hole”.
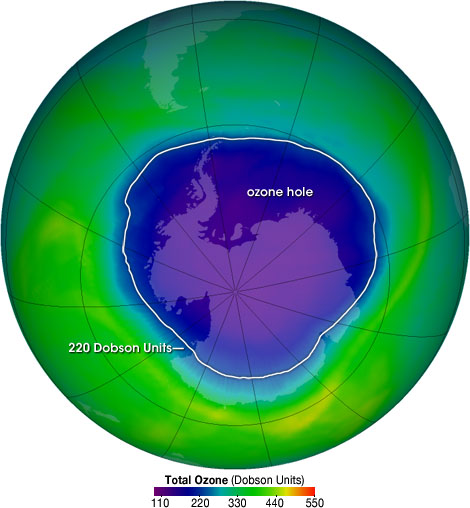
The area of the ozone hole is determined from a map of total column ozone. It is calculated from the area on the Earth that is enclosed by a line with a constant value of 220 Dobson Units. The value of 220 Dobson Units is chosen since total ozone values of less than 220 Dobson Units were not found in the historic observations over Antarctica prior to 1979. Also, from direct measurements over Antarctica, a column ozone level of less than 220 Dobson Units is a result of the ozone loss from chlorine and bromine compounds.
What is DOBSON UNIT?
The Dobson Unit (DU) is the unit of measure for total ozone. If you were to take all the ozone in a column of air stretching from the surface of the earth to space, and bring all that ozone to standard temperature (0 °Celsius) and pressure (1013.25 millibars, or one atmosphere, or “atm”), the column would be about 0.3 centimeters thick. Thus, the total ozone would be 0.3 atm-cm. To make the units easier to work with, the “Dobson Unit” is defined to be 0.001 atm-cm. Our 0.3 atm-cm would be 300 DU.
Click on image to start animation
Chlorofluorocarbons and ozone
CFCs escape into the atmosphere from refrigeration and propellant devices and processes. In the lower atmosphere, they are so stable that they persist for years, even decades. This long lifetime allows some of the CFCs to eventually reach the stratosphere. In the stratosphere, ultraviolet light breaks the bond holding chlorine atoms (Cl) to the CFC molecule. A free chlorine atom goes on to participate in a series of chemical reactions that both destroy ozone and return the free chlorine atom to the atmosphere unchanged, where it can destroy more and more ozone molecules. For those who know the story of CFCs and ozone, that is the part of the tale that is probably familiar.
The part of the story that fewer people know is that while the chlorine atoms freed from CFCs do ultimately destroy ozone, the destruction doesn’t happen immediately. Most of the roaming chlorine that gets separated from CFCs actually becomes part of two chemicals that—under normal atmospheric conditions—are so stable that scientists consider them to be long-term reservoirs for chlorine.

Polar stratospheric clouds and ozone
Under normal atmospheric conditions, the two chemicals that store most atmospheric chlorine (hydrochloric acid, and chlorine nitrate) are stable. But in the long months of polar darkness over Antarctica in the winter, atmospheric conditions are unusual. An endlessly circling whirlpool of stratospheric winds called the polar vortex isolates the air in the center. Because it is completely dark, the air in the vortex gets so cold that clouds form, even though the Antarctic air is extremely thin and dry. Chemical reactions take place that could not take place anywhere else in the atmosphere. These unusual reactions can occur only on the surface of polar stratospheric cloud particles, which may be water, ice, or nitric acid, depending on the temperature. These reactions convert the inactive chlorine reservoir chemicals into more active forms, especially chlorine gas (Cl2). When the sunlight returns to the South Pole in October, UV light rapidly breaks the bond between the two chlorine atoms, releasing free chlorine into the stratosphere, where it takes part in reactions that destroy ozone molecules while regenerating the chlorine (known as a catalytic reaction). A catalytic reaction allows a single chlorine atom to destroy thousands of ozone molecules. Bromine is involved in a second catalytic reaction with chlorine that contributes a large fraction of ozone loss. The ozone hole grows throughout the early spring until temperatures warm and the polar vortex weakens, ending the isolation of the air in the polar vortex. As air from the surrounding latitudes mixes into the polar region, the ozone-destroying forms of chlorine disperse. The ozone layer stabilizes until the following spring.
Here you can find great multimedia resources that explain Ozone layer, it’s chemistry and depletion layer that makes hole.

WMO/UNEP Scientific Assessments of Ozone Depletion
The Ozone Hole Tour
Ozone Monitoring Instrument

Commenting rules and guidelines
We value the thoughts and opinions of our readers and welcome healthy discussions on our website. In order to maintain a respectful and positive community, we ask that all commenters follow these rules.