A breakthrough in understanding the secret life of prion molecules
New research from David Westaway, PhD, of the University of Alberta and Jiri Safar, PhD, Case Western Reserve University School of Medicine has uncovered a quality control mechanism in brain cells that may help keep deadly neurological diseases in check for months or years.
The findings, published in The Journal of Clinical Investigation, "present a breakthrough in understanding the secret life of prion molecules in the brain and may offer a new way to treat prion diseases," said Westaway, Director of the Centre for Prions and Protein Folding Diseases and Professor of Neurology in the Faculty of Medicine and Dentistry at the University of Alberta.
Prion diseases lead to incurable neurodegenerative disorders such as Creutzfeldt-Jakob disease in humans, mad cow disease (Bovine Spongiform Encephalopathy) and chronic wasting disease in deer and elk. The diseases are caused by the conversion of normal cellular prion proteins into the diseased form.
For years, scientists have been perplexed by two unexplained characteristics of prion infections: vastly differing asymptomatic periods lasting up to five decades and when symptoms do arise, greatly varying accumulation of the diseased proteins. In striking contrast, test tube prions replicate rapidly, and in a matter of days reach levels found in brains in the final stage of the disease.
"Our study investigated the molecular mechanism of this intriguing puzzle," said Safar, Co-Director of the National Prion Disease Pathology Surveillance Center and Associate Professor in Departments of Pathology and Neurology in Case Western Reserve University School of Medicine.
In probing these mysteries, Westaway, Safar, their teams and other collaborating researchers in the U.S., Italy and the Netherlands studied a molecule called the 'shadow of the prion protein.'
"Dramatic changes in this shadow protein led us to expand our view to include the normal prion protein itself," said Westaway. "This is a crucial molecule in brain cells because it is pirated as the raw material to make diseased prion proteins."
The production and degradation of the normal prion protein had previously received little attention because it was assumed its production pipeline did not vary.
"The puzzle of the long asymptomatic time period required sorting out the different types of prion protein molecules. Our laboratory developed new techniques to tease out these subtle differences in shape," Safar said.
The researchers discovered a marked drop in the amount of the normal prion protein in eight different types of prion diseases. Strikingly, this drop occurred months or years before the animal models showed tell-tale clinical symptoms of the brain disease.
"Our belief is that cells under prion attack are smarter than we once thought," Westaway said. "They not only sense the molecular piracy by the diseased proteins, but they also adopt a simple and at least partly effective protective response – they minimize the amount raw material from the pipeline for prion production."
"We believe we can kill two birds with one stone, because the normal prion protein is also a receptor for toxicity. Augmenting this natural protective response may be a preferred route to cure prion infections," Safar added.
The study's discovery of a natural protective response can also explain the long latency period in other more common neurodegenerative diseases.
"The pre-clinical phase of the disease—before it shows symptoms—is when you want to set things straight. We may be able to take a slow disease and bring it to a complete standstill," Westaway said. "Since some scientists believe the normal prion protein is an accessory in the brain cell death of Alzheimer's disease, gaining a new understanding of rare yet lethal prion diseases may provoke fresh insights into human dementias."
Source: University of Alberta
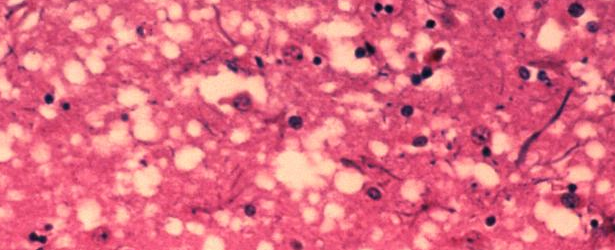
Featured image: This micrograph of brain tissue reveals the cytoarchitectural histopathologic changes found in bovine spongiform encephalopathy. The presence of vacuoles, i.e. microscopic “holes” in the gray matter, gives the brain of BSE-affected cows a sponge-like appearance when tissue sections are examined in the lab. Image credit: Public Health Image Library, APHIS. Author: Dr. Al Jenny.

Commenting rules and guidelines
We value the thoughts and opinions of our readers and welcome healthy discussions on our website. In order to maintain a respectful and positive community, we ask that all commenters follow these rules.