Scientists discover highly efficient way of producing hydrogen
Researchers from the University of Houston and the California Institute of Technology have discovered an efficient catalyst that uses molybdenum sulfoselenide particles on three-dimensional porous nickel diselenide foam to increase catalytic activity. The step is regarded as an important breakthrough in finding and utilizing a practical approach for producing large amounts of hydrogen gas, known as the fuel of the future.
Hydrogen, in the shape of fuel cells, is regarded as the fuel for the future, with the capability to power electric motors. However, finding a practical, inexpensive and nontoxic way for producing large amounts of hydrogen gas, has proven a challenge for the scientific community.
The discovered foam, produced with commercially available nickel foam, has significantly affected the catalytic performance, as it exposed more edge sites with catalytic activity reported higher than on flat surfaces.
The foam, made using commercially available nickel foam, significantly improved catalytic performance because it exposed more edge sites, where catalytic activity is higher than it is on flat surfaces, said Zhifeng Ren, MD Anderson Professor of physics at UH.
"With the massive consumption of fossil fuels and its detrimental impact on the environment, methods of generating clean power are urgent. Hydrogen is an ideal carrier for renewable energy; however, hydrogen generation is inefficient because of the lack of robust catalysts that are substantially cheaper than platinum," explained the scientists.
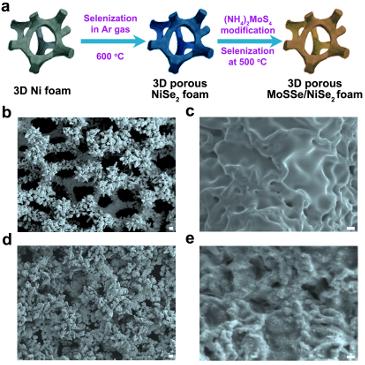
Illustration shows procedures for growing ternary molybdenum sulfoselenide on the porous foam; b-c, images showing surface roughness of the nickel diselenide foam grown at 600 degrees C; d-e, morphologies of ternary molybdenum sulfoselenide particles on porous foam, grown at 500 °C.
According to Zhifeng Ran, MD Anderson Professor of physics at UH and a principal investigator at the Texas Center for Superconductivity, the platinum catalysts, have reported highest efficiency rates for hydrogen evolution. However, platinum is rare, difficult to extract and highly expensive for practical use, and therefore the scientists will continue to search for more efficient ways of spliting water into the component parts.
At present, hydrogen is produced by steam methane reforming and coal gasification, the methods which raise the fuel's carbon footprint.
Molybdenum sulfoselenide and similar layered compounds are regarded as promising catalysts, although no one has boosted their performance to viable levels in bulk form, so far. The most active catalysis on those layered compounds, the layered transition-metal dichalcogenides, or LTMDs, take place at the edges, which makes the idea of a substrate with a large number of exposed edges more desirable.
Their hybrid catalyst is composed of molybdenum sulfoselenide particles with vertically aligned layers on a 3-D porous conductive nickel diselenide scaffold.
The hybrid catalyst requires 69 millivolts from an external energy source to achieve a current density of 10 milliamps per square centimeter, a result much better than previously conducted tests. The current "splits" the water, converting it to hydrogen at the cathode. Achieving the necessary current density with lower voltage improves energy conversion efficiency and reduces preparation costs.
A platinum catalyst required 32 millivolts and ongoing testing has reduced the hybrid catalyst requirements to about 40 millivolts, close to the platinum requirements. According to the scientists, the ability to increase current output at a faster rate than the increase in required energy input is equally important. The catalyst remained stable after 1 000 cycles at a constant current.
Featured image: Illustration shows procedures for growing ternary molybdenum sulfoselenide on the porous foam; b-c, images showing surface roughness of the nickel diselenide foam grown at 600 degrees C; d-e, morphologies of ternary molybdenum sulfoselenide particles on porous foam, grown at 500 °C.

Commenting rules and guidelines
We value the thoughts and opinions of our readers and welcome healthy discussions on our website. In order to maintain a respectful and positive community, we ask that all commenters follow these rules.